Carboxymethyl Cellulose (CMC) and steroids are distinctly different in terms of their chemical structure, biological function, and applications. To clarify this difference, it’s essential to delve into the basics of each compound, their chemical characteristics, and their roles in various industries.
Understanding Carboxymethyl Cellulose (CMC)
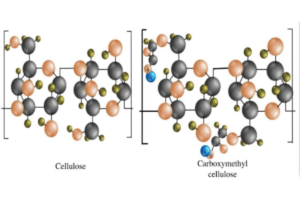
- Chemical Structure: CMC is a derivative of cellulose, which is a natural polysaccharide made up of long chains of glucose molecules linked together. In CMC, some hydroxyl groups (-OH) of the cellulose are substituted with carboxymethyl groups (-CH2-COOH). This modification makes CMC water-soluble, a property not typically found in native cellulose.
- Production Process: CMC is synthesized through a process called carboxymethylation, where cellulose reacts with sodium hydroxide and chloroacetic acid. This reaction replaces some of the hydroxyl groups in cellulose with carboxymethyl groups.
- Properties and Uses: CMC is known for its ability to increase the viscosity of solutions, act as a stabilizer, and improve texture. It’s widely used in the food industry as a thickener, in pharmaceuticals as a binder and stabilizer, and in personal care products for its texture-enhancing properties.
- Non-Steroidal Nature: CMC, being a cellulose derivative, is a carbohydrate. It does not possess the defining structural characteristics of steroids, which are essentially lipids (fats).
Understanding Steroids
- Chemical Structure: Steroids are a class of organic compounds characterized by a core structure of four linked carbon rings. This structure is fundamentally different from the long-chain carbohydrate structure of CMC.
- Types and Functions: Steroids include compounds such as cholesterol, sex hormones like testosterone and estrogen, and corticosteroids. These compounds play diverse roles in the body, from structural components of cell membranes (cholesterol) to signaling molecules that regulate various physiological processes (hormones).
- Biological Importance: Steroids are critical for many bodily functions, including metabolism, immune response, and the development of sexual characteristics. They are synthesized naturally in the body and can also be manufactured synthetically for medical use.
Key Differences Between CMC and Steroids
- Chemical Composition: CMC is a modified polysaccharide, a carbohydrate with a structure vastly different from the lipid-based four-ring structure of steroids.
- Function and Use: CMC is used for its physical properties like viscosity and stability, particularly in industrial applications. Steroids, on the other hand, are involved in vital biological functions and are used medically to treat a range of conditions, from hormonal imbalances to inflammation.
- Synthesis and Source: CMC is synthesized from cellulose, primarily derived from plants, whereas steroids are naturally synthesized in the bodies of animals, including humans.
- Health Implications: While steroids can have significant physiological effects and potential side effects, CMC is generally considered safe and is used primarily for its functional properties in various products.
| Feature | Carboxymethyl Cellulose (CMC) | Steroids |
|---|---|---|
| Chemical Classification | Polysaccharide (Carbohydrate) | Lipid (Fat) |
| Chemical Structure | Long chains of glucose molecules with carboxymethyl groups | Four linked carbon rings |
| Source | Derived from plant-based cellulose (wood pulp, cotton lint) | Naturally produced in animal bodies; can also be synthetically manufactured |
| Primary Use | Thickener, stabilizer, and texture enhancer in food, pharmaceuticals, and cosmetics | Hormonal regulation, anti-inflammatory agents, key component in cell membranes |
| Production Process | Synthesized through carboxymethylation of cellulose | Biosynthesized in the body from cholesterol; also industrially synthesized |
| Biological Role | None, used for physical properties in products | Critical for various physiological processes like metabolism, immune response, and development of sexual characteristics |
| Health Implications | Generally considered safe and non-toxic, used in a wide range of consumer products | Can have significant physiological effects and potential side effects; used in medicine for specific conditions |
| Solubility | Water-soluble (especially when modified) | Typically fat-soluble |
Conclusion
In conclusion, Carboxymethyl Cellulose (CMC) is not a steroid. It is a chemically modified carbohydrate with a structure and purpose fundamentally different from steroids. Understanding these differences is crucial, especially in fields like pharmacology, nutrition, and food science, where the specific properties and functions of these compounds are critical. CMC’s role as a non-toxic, versatile additive in various industries stands in contrast to the complex biological functions and medical applications of steroids.